Balanced Chemical Equations Imply Which of the Following
Here number of chlorine and hydrogen atoms on the reactant and product side are not same. The balanced equation will appear above.

Nh2oh Lewis Structure Hydroxylamine Math Lewis Chemical Formula
Due to the fact that each facet is well balanced the balanced chemical equation is.
. A chemical reaction in which two or more substances undergo a chemical union to form a. D b and c E a and b. Mass is conserved in chemical change.
If there would be no coefficient in front of a given compound in the balanced equation just write type 1 C2H2. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. Lets take an example of a balanced chemical equation eg K2CO3aq2HClaq 2KClaqCO2gH2Ol K 2 C O 3 a q 2 H C l a q 2 K C l a q C O 2 g H 2.
Which of the following equations is NOT balanced. Numbers of atoms are conserved in chemical change. Balancing Chemical Equations Using MINOH One of the most useful devices for communicating information related to chemical changes is the chemical equation.
In balancing an equation we change the to make the number of atoms on each side of the equation balanced. Numbers of atoms are conserved in chemical change. Balanced chemical equations imply which of the following.
How many of the following are true concerning balanced chemical equations. Volume is conserved in chemical change d. C Volume is conserved in a chemical change.
Numbers of atoms are conserved in chemical change. See below for the correct answer. Al 2 SO 4 3 Ca OH 2 Al OH 3 CaSO 4 Selected Answer.
The coefficients indicate the mass ratios of the substances used. A and b e. Numbers of molecules are conserved in chemical change.
Numbers of atoms are conserved in chemical change. Balanced chemical equations imply which of the following. The measure of the number of atoms in one element that will combine with an atom of another element is ValenceUser.
Consider the following chemical equation. 100 correct and accurate. C2H2 g O2 g CO2 g H2O g What would be the coefficients in front of each reactant and product in the balanced equation.
Volume is conserved in chemical change. A and B E. Numbers of atoms are conserved in chemical change.
CUS HNO3aq CuNO32aq 4H2OI NOg C. Balanced chemical equations imply which of the following. Which of the following equations is NOT balanced.
Numbers of molecules are conserved in chemical change II. Ionic charges are not yet supported and will be ignored. Fe Au Co Br C O N F.
Numbers of atoms are conserved in chemical change. Balanced chemical equations imply which of the following. See the answer See the answer done loading.
Balanced chemical equations imply which of the following. Balanced chemical equations imply which of the following. Balanced chemical equations imply which of the following.
Balanced chemical equations imply which of the following. Numbers of molecules are conserved in chemical change. Numbers of molecules are conserved in chemical change.
The correct answer is They provide the molar ratio of reactant and products in a chemical reaction. B Numbers of atoms are conserved in chemical change. B Numbers of atoms are conserved in a chemical change.
Atoms are neither created nor destroyed IV. Volume is conserved in chemical change. Balanced chemical equation helps us to calculate the number of moles of both reactant and productFor example 2H2O22H2O From the above balanced equation it can be stated that 2 molecules of hydrogenH2 reacts with 1 molecule of.
Question 3 1 out of 1 points Balance the following equation. Volume is conserved in chemical change. Question 2 1 out of 1 points When the following equation is balanced what is the sum of the coefficients.
A chemical reaction in which two or more substances undergo a chemical union to form a more complex substance is a _ reaction Weegy. CH4g 2029 CO2g 2H2Og B. The coefficients for the reactants tell you how much of each reactant you are given III.
Every balanced chemical equation follows law of conservation of mass. Use uppercase for the first character in the element and lowercase for the second character. D B and C 2.
Numbers of molecules are conserved in chemical change b. It may also include the energy. A Numbers of molecules are conserved in chemical change.
In other words the mass and the charge are balanced on both sides of the reaction. The number of molecules is conserved II. B 2 O 3 s HF l BF 3 g H 2 O l Selected.
The equation contains both qualitative and quantitative information related to the nature and quantity of the substances involved in the chemical reaction. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. - Which of the following equations is NOT balanced.
Thus this equation is not balanced. Numbers of atoms are conserved in chemical change c. A Numbers of molecules are conserved in a chemical change.
2KCIO3s - Past Question and answers for schoolworks. Volume is conserved in chemical change. For the given options.
Numbers of molecules are conserved in chemical change. Chemistry questions and answers. Here number of chlorine and bromine atoms on the reactant and product side are not same.
C Volume is conserved in chemical change.
What Are Balanced Chemical Equations Quora
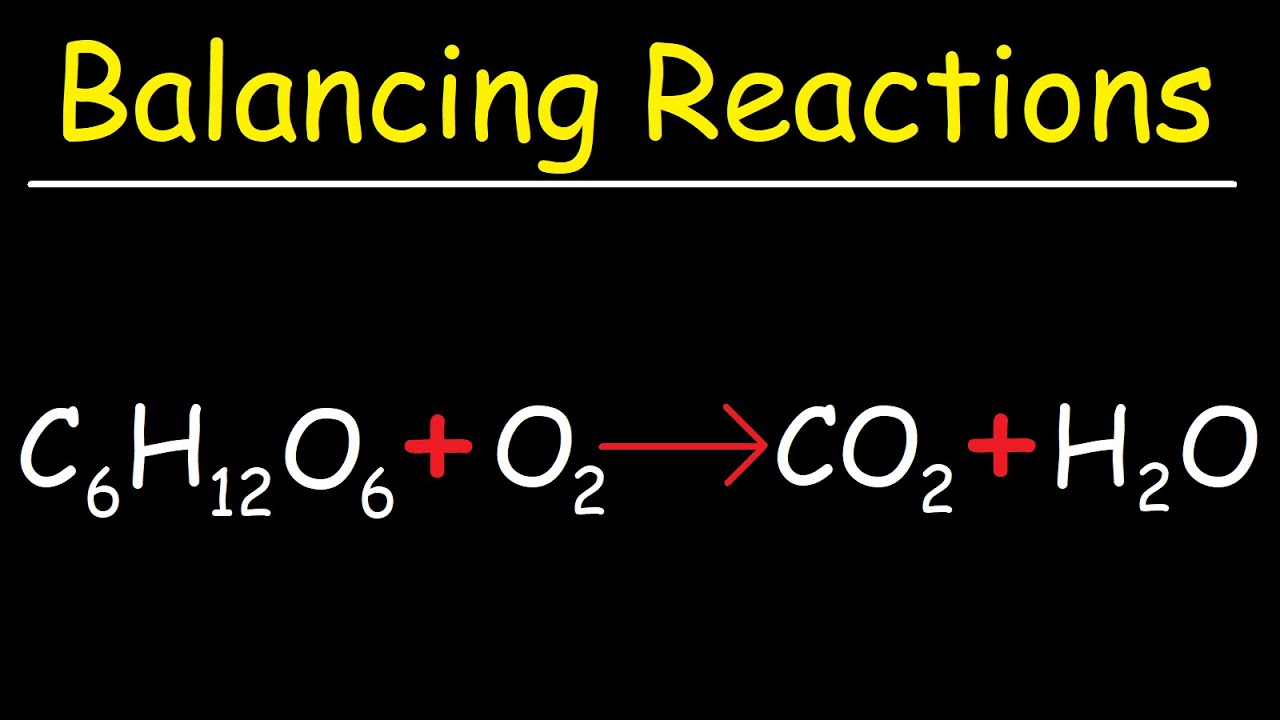
How To Balance C6h12o6 O2 Co2 H2o Cellular Respiration Photosynthesis Youtube

Correlation Vs Causation A Mathographic Teaching Psychology Ap Psychology Statistics Math
No comments for "Balanced Chemical Equations Imply Which of the Following"
Post a Comment